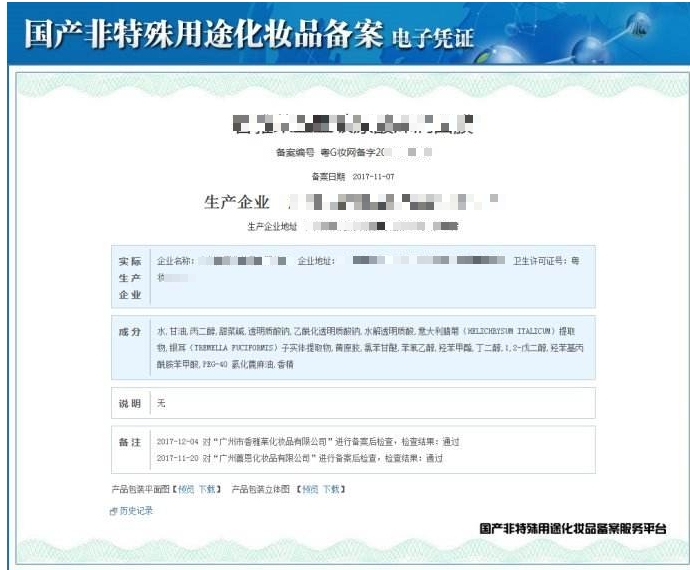
# Domestic non-special use cosmetics information filing
Provisions on the Recordation of Domestic Non-Special-purpose Cosmetics
(1) For any non-special-purpose cosmetics produced within the territory of the People's Republic of China, the production enterprises shall conduct product information recordation in accordance with the requirements of these Provisions.
II. A production enterprise shall, before a product is put on the market for sale, make the product formula (excluding content, except for restricted substances) and sales packaging (including product labels, The information of product instructions shall be submitted to the provincial food and drug regulatory authorities in the administrative region through a unified network platform as required. Product safety assessment data, product production process description, product production equipment list, Product technical requirements and product inspection reports shall be kept by the production enterprise for future reference.
You can go to the National Drug Administration: http://ftba.nmpa.gov.cn:8181/ftban/fw.jsp